

- Who arranged the elements according to atomic mass series#
- Who arranged the elements according to atomic mass free#
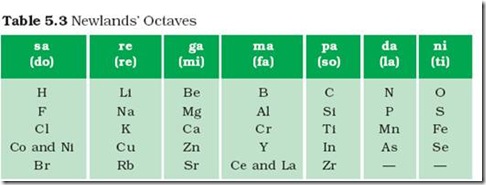
The most distinctive property of the noble gases is that they are. Which orbital (s, p, d, f) of the following elements is filling?Ī. Indicate which is the largest in each pair. Indicate which element in each pair has the lowest ionization energy. Indicate which element in each pair has the smaller atomic radius. Why do the noble gases lack electronegativity values?īecause they don't have a desire to gain electron Why does Chlorine have a higher ionization energy than Sulfur?īecause Chlorine only needs 1 electron to become full, but Sulfur needs 2 so chlorine has more desire to gain an electron. Rank the following elements form high to low electronegativity: Carbon, Aluminum, Oxygen, Potassium.ġ. Rank the following elements by increasing atomic radius: Sulfur, Oxygen, Neon, Aluminum Low ionization energy is characteristics of: Which of the following is most likely to gain electrons in a chemical reaction? Which of the following has a smaller radius? Which of the following elements is least metallic? What is the most probable oxidation state of the alkaline earth metals? Which of the following influenced your answer the most? The principle that states that the physical properties of the elements are periodic functions of their atomic numbers is.įrom which of the following is it easiest to remove and electron? What are the radioactive elements with atomic numbers form 90 to 103 called? The discovery of what elements added a new column to Mendeleev's periodic table? permits the properties of an element to be predicted before the element is discovered. was completed with the discovery of the nobleĪ.

has been of little use to chemists since the early 1900s D. permits the properties of an element to be predicted before the element is discovered B. Mendeleev noticed that properties of elements usually repeated at regular intervals when the elements were arranged in order of increasing. The idea of arranging the elements in the periodic table according to their chemical and physical properties is attributed to.
Who arranged the elements according to atomic mass free#
As with Chemistry, the text of Wikipedia is available under the GNU Free Documentation License.How did Dmitri Mendeleev arrange the elements on his periodic table? The list of authors can be seen in the page history. The original article was at List of elements by atomic mass. Series: Alkalis - Alkaline earths - Lanthanides - Actinides - Transition metals - Poor metals - Metalloids - Nonmetals - Halogens - Noble gasesīlocks: s-block - p-block - d-block - f-block - g-block Name | Atomic symbol | Atomic number | Boiling point | Melting point | Density | Atomic mass Standard table | Vertical table | Table with names | Names and atomic masses (large) | Names and atomic masses (small) | Names and atomic masses (text only) | Inline F-block | Elements to 218 | Electron configurations | Metals and nonmetals | Table by blocks | Alternatives Atomic weights of elements with atomic numbers 110-116 taken from this source. IUPAC Standard Atomic Weights Revised (2005).Atomic weights of elements with atomic numbers from 1-109 taken from this source. Atomic Weights of the Elements 2001, Pure Appl.Note 5: The atomic weight of commercial Lithium can vary between 6.939 and 6.996-analysis of the specific material is necessary to find a more accurate value.Note 4: The isotopic composition varies in terrestrial material such that a more precise atomic weight can not be given.Note 3: The isotopic composition of the element can vary in commercial materials, which can cause the atomic weight to deviate significantly from the given value.Note 2: The isotopic composition of this element varies in some geological specimens, and the variation may exceed the uncertainty stated in the table.However, three elements, Thorium, Protactinium, and Uranium, have a characteristic terrestrial isotopic composition, and thus their atomic mass given. , indicates the mass number of the longest-lived isotope of the element. Note 1: The element does not have any stable nuclides, and a value in brackets, e.g.
Who arranged the elements according to atomic mass series#
For artificial elements the nucleon count of the most stable isotope is listed bracketsĬhemical series of the periodic table Alkali metals
The number in parenthesis gives the uncertainty in the "concise notation" dis given in parenthesis next to the least significant digits to which it applies", e.g., 1.00794(7) stands for 1.00794 ± 0.00007. Each element's atomic number, name, element symbol, and group and period numbers on the periodic table are given. This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element.


 0 kommentar(er)
0 kommentar(er)
